好消息? RECEIPT OF PRC REGULATORY APPROVALS FOR COMMERCIAL PRODUCTIONhttp://info.sgx.com/webcoranncatth.nsf/VwAttachments/Att_B0EB1DBA47B6E9C6482577C700360CA1/$file/CAH_SGXAnn_BiweiAntai_PRCApprovalVaccine.pdf?openelement
- RECEIPT OF PRC REGULATORY APPROVALS FOR COMMERCIAL PRODUCTION
AND SALE OF ANIMAL FOOT-AND-MOUTH DISEASE (“FMD”) VACCINE
The Directors of the Company are pleased to inform shareholders that the Company’s
subsidiary, Biwei Antai, has received regulatory approvals from the PRC Ministry of
Agriculture (“MOA”) to commence commercial production and sale of its FMD vaccines. The
administrative issuance of the relevant drug numbers by MOA is expected to follow in due
course.
With the receipt of such approvals, the Company will be able to participate in tenders for the
purchase of such vaccines which are called by regional government animal disease control
centres in the PRC.
好消息!

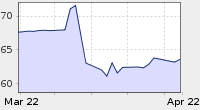
Oil Price:
 .
.

 Huasing Association 1999 - 2013
Huasing Association 1999 - 2013